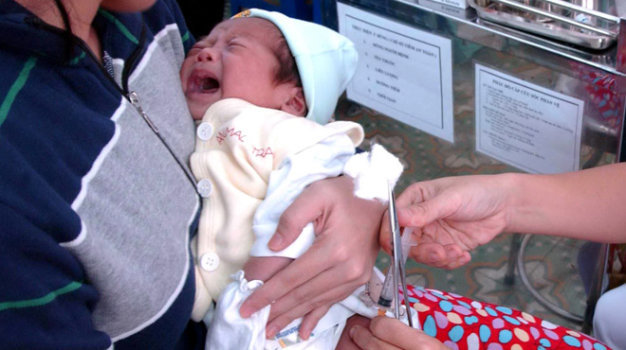
Many Vietnamese parents are now bringing their children to Singapore for costly vaccinations for fear of unsafe free-of-charge Quinvaxem vaccine along with a shortage in supply of the substitute vaccine, which is expensive but believed to be safer.
The Korean-made Quinvaxem vaccine is suspected to have caused the deaths of many children in Vietnam over recent years.
Overseas vaccination tours, which have recently been established and organized by a Vietnamese family living in Singapore, is gaining popularity among Vietnamese families, according to Sai Gon Tiep Thi newspaper.
A four-day three-night tour to Singapore costs a total of VND16 million (US$713) and covers all expenses, including transportation, accommodation, translated consultation, diagnosis and an injection.
Despite the high price, more and more Vietnamese parents are willing to spend big to ensure the safety of their children, preferring to boycott the controversial but commonly used and free-of-charge Quinvaxem vaccine.
The vaccine is meant to prevent five common, potentially fatal childhood diseases: diphtheria (D), tetanus (T), pertussis (P, whooping cough), hepatitis B (HepB), and Haemophilus influenza type b (Hib).
It was included in the National Expanded Immunization Program in June 2010.
The use of the vaccine was halted for five months at the beginning of May 2013 after nine children died shortly after being vaccinated between December 2012 and March 2013.
Its use was resumed on October 2013 after investigations by Vietnamese health authorities showed that there were no unsafe cases in relation to the use of this vaccine.
The number of children who have died after being injected with the Quinvaxem vaccine was 61 in 2013, according to statistics provided by the Vietnamese Ministry of Health.
There have been 16 cases in which children had a severe allergic reaction to the vaccination, of which eight resulted in fatalities, in 2015 alone, the ministry said.
Safety guarantee from WHO, UNICEF
In December 2013, the World Health Organization (WHO) and UNICEF jointly confirmed that the 5-in-1 Quinvaxem vaccine was safe to use after carrying out an investigation purported to have shown that it meets all of the highest international safety standards.
There are strict quality controls at every step of the manufacturing and supply processes and the vaccine is prequalified to assure its quality in accordance with the highest global standards, the WHO said. Like all vaccines, Quinvaxem can have some mild side effects, including low grade fever, localized swelling, and general short-term discomfort, the WHO and UNICEF said.
In a very rare number of cases – less than one per million – a child can have a severe allergic reaction to the vaccine, they added.
Health care providers are trained to respond and if prompt medical treatment is given, the child can make a full recovery, the two said.
Supported by Vietnamese government
Quinvaxem vaccine remains applied in the National Expanded Immunization Program in which infants between two and four months old are injected with a free vaccination, Vietnamese Minister of Health Nguyen Thi Kim Tien said on November 2.
The rate of children with an allergic reaction to the vaccine in Vietnam is 4.5 in a million injections, much lower than the recommended rate given by the producers as well as the WHO, according to Dr. Tran Dac Phu, head of the General Department of Preventive Medicine under the health ministry.
High demand for alternative methods
Despite Quinvaxem being endorsed by the WHO and Vietnamese government, many Vietnamese families prefer the substitute vaccine called Pentaxim, which is not free but assumed to be safer.
A recent surge in demand for the paid vaccination has caused the vaccine to become out of stock.
In spite of the considerably high cost, which has risen to VND692,000 ($30.8) per shot this year, the local supplier still cannot meet domestic demand as most parents want to ensure their children’s safety.
The shortage in the Pentaxim supply is expected to continue till the end of 2015 and throughout 2016, increasing the risk of children being infected with fatal diseases, according to the Vietnamese Ministry of Health.
The decline in supply is due to the producing companies’ change in production technologies, procedures and positions, the ministry explained.
Pentaxim will be prioritized for the nations in which the vaccine is used in the National Expanded Immunization Program, it elaborated.
Like us on Facebook or follow us on Twitter to get the latest news about Vietnam!