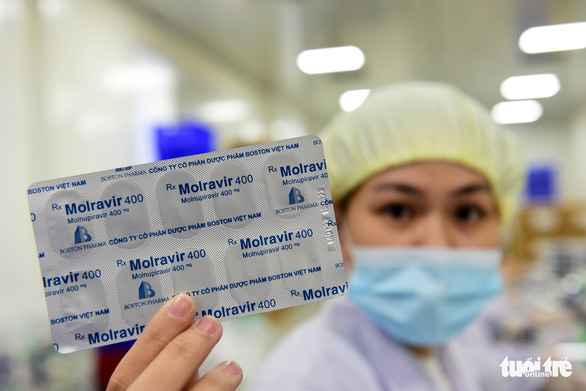
Three domestic companies are boosting manufacturing of antiviral drugs against COVID-19 to provide the medications at a price lower than that recommended by the World Health Organization (WHO), after obtaining licenses from Vietnam’s Ministry of Health.
The firms are Boston Vietnam Pharmaceutical Joint Stock Company (Boston Pharma), Stellapharm Joint Venture Co. Ltd. (Stellapharm), and Mekophar Chemical Pharmaceutical Joint Stock Company (Mekophar), which have prepared Molravir 400mg, Molnupiravir Stella 400mg, and Movinavir 200mg, respectively.
They were licensed by the ministry on Thursday to manufacture and distribute COVID-19 antiviral pills containing the active ingredient Molnupiravir, with their licenses to be effective for three years.
|
|
| This photo shows workers producing Molravir 400mg at a factory of Boston Vietnam Pharmaceutical Joint Stock Company in Binh Duong Province, Vietnam. Photo: Duyen Phan / Tuoi Tre |
The three drugs have a shelf life of six to eight months and come in the form of hard capsules.
Binh Duong Province-based Boston Pharma has a production capacity of 30 million pills per month and is awaiting opinions from agencies concerned about pricing before launching its products on the market, said Luong Dang Khoa, the firm’s general director, told Tuoi Tre (Youth) newspaper.
Following research and development since September 2021, during which Boston Pharma faced different difficulties including costly input materials, the company completed its first Molravir 400mg on January 5, 2022, Khoa said.
The company expects to offer Molvarir 400mg to pharmacies at the original price of VND273,000 (US$11.9) per box (inclusive of VAT), much lower than the WHO-recommended price of $19.9 intended for less developed countries, Khoa stated.
More than 10,000 drugstores nationwide have signed official contracts to buy Molvarir 400mg from Boston Pharma and they will then offer it to the public for about VND300,000 ($13.1) per box.
Meanwhile, a representative of Ho Chi Minh City-based Stellapharm Joint Venture Co. Ltd. said that its product, Molnupiravir Stella 400mg, meets the European Union’s GMP (good manufacturing practice) standards while complying with the requirements registered with the Drug Administration of Vietnam under the health ministry.
With its strict quality management system, the company is committed to ensuring medicine with stable quality.
According to reports published by the ministry, the drug is safe, effective, and well-tolerated in treating COVID-19 patients, as it helps reduce viral load and transmission, prevent severity, and shorten treatment time, the company said.
Stellapharm is coordinating with its distributors to offer its products to consumers for VND250,000 ($10.9) per box of 20 pills.
|
|
| This image shows a batch of finished Molravir 400mg pills at Binh Duong Province-based Boston Vietnam Pharmaceutical Joint Stock Company. Photo: Duyen Phan / Tuoi Tre |
“In order to ensure the interests of the patients, we will coordinate with distribution units to make sure that the medicine is provided at the same price across the country,” the firm’s representative affirmed.
Another approved COVID-19 antiviral drug is Movinavir 200mg, whose efficacy has been proven as fairly good through research and testing, according to its manufacturer, Mekophar, based in Ho Chi Minh City.
“Patients will not fall into severe conditions and will retest negative after three to five days of taking this drug,” said Huynh Thi Lan, general director of Mekophar.
Lan also affirmed that Movinavir 200mg will be sold “at the best possible price for the sake of people.”
The company’s leader expressed her regret that the antiviral drugs domestically manufactured have been approved and licensed for circulation quite slowly, causing people to buy the medicine of unknown origin.
As Tuoi Tre has found, many large drugstore chains, including one that has more than 500 pharmacies nationwide, have signed deals to buy the antiviral drugs from Vietnamese pharmaceutical firms.
Since the pandemic erupted in Vietnam in early 2020, the Southeast Asian country has documented 2,890,252 COVID-19 cases, with 2,305,081 recoveries and 39,682 fatalities, the health ministry reported on Tuesday.
Like us on Facebook or follow us on Twitter to get the latest news about Vietnam!